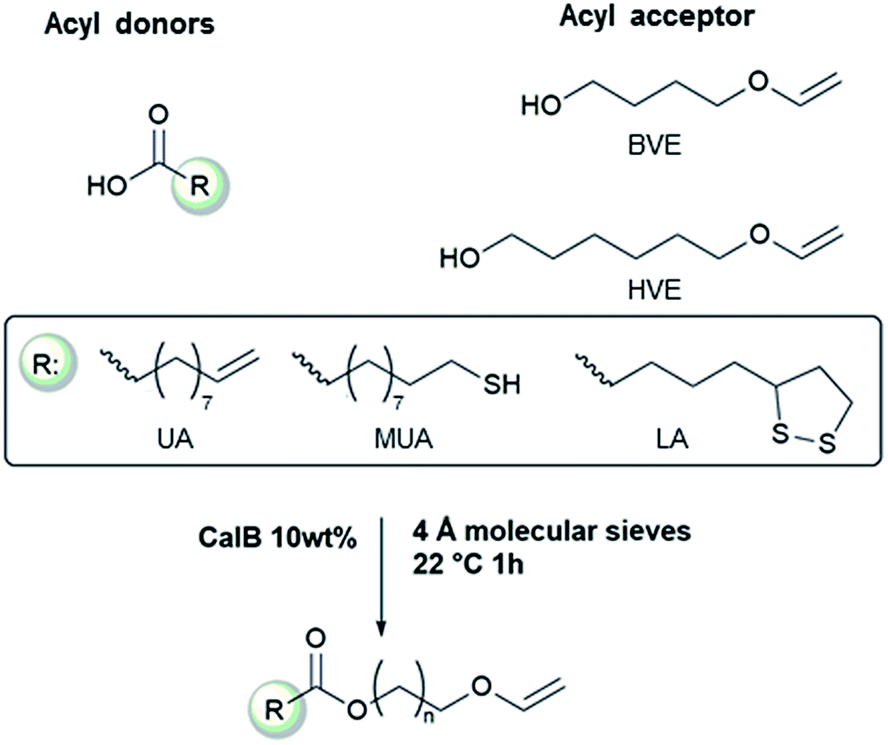
Inhibitors such as mehq monomethyl ether hydroquinone and ptz phenothiazine are added to acrylic acid in the shipping and storage process to prevent its spontaneous polymerization.
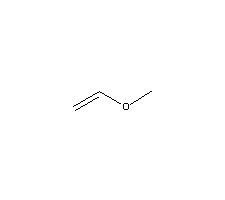
Vinyl ether acrylic acid.
It is the simplest unsaturated carboxylic acid consisting of a vinyl group connected directly to a carboxylic acid terminus.
This colorless liquid has a characteristic acrid or tart smell.
They are increasingly used in radiation curing systems because of a lower toxicity profile than the commonly used acrylic monomers.
Dissolved oxygen is also an strong inhibitor and its presence in the solution enhances the inhibition effects of mehq.
Acrylic elastomer can generally be characterized as one of two types.
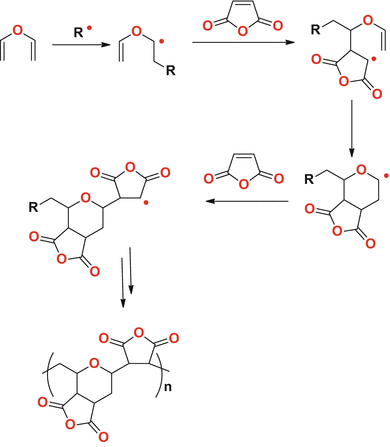
Vinyl ethers undergo homopolymerization via a cationic mechanism.
Ch2 cr1 coo r2 0 ch ch r3 1 in the formula r1 represents a hydrogen atom or a methyl group r2 represents an organic.
Literature values for the glass transition temperature t g and melting temperature t m for the more common homopolymers are listed in the table below polymers are listed by the repeating unit in the polymer chain.
Phosphate buffer solution pbs of ph 7 4 was used as swelling medium in order to determine the temperature.
A process is disclosed for the preparation of acrylic acid precursors by a hydroformylation process which comprises reacting a vinyl ether with carbon monoxide and hydrogen in the presence of a catalyst comprising a rhodium carbonyl compound and a phosphine ligand at a mild temperature and pressure until there is substantial formation of the intermediate 2 and 3 ethoxypropanals followed by.
Even traces of.
The polymers and corresponding monomers listed are available from us.
A vinyl ether group containing meth acrylic ester composition which comprises a radical polymerization inhibitor and a vinyl ether group containing meth acrylic ester represented by the following general formula 1.
In this work we developed a comprehensive mathematic model for the inhibition of acrylic.
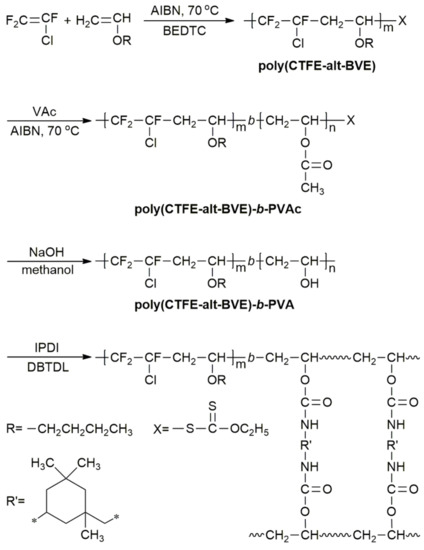
21 the vinyl ether transfer agents like other vinyl ethers generally show marked acid sensitivity and are not suited for use with acidic monomers e g acrylic acid aa methacrylic acid maa.
Functionality can be introduced on z or r to modify reactivity or to tailor the end groups as in the examples 20 22.
Vinyl ethers undergo radical initiated copolymerization in the presence of specific monomers such as maleates fumarates and acrylics.
Propenoic acid is an organic compound with the formula ch 2 chcooh.
It is miscible with water alcohols ethers and chloroform more than a million tons are produced annually.