Marta meazza ramon rios cheminform abstract.
Vinyl cyclopropane synthesis.
Co p1 is an effective catalyst for asymmetric cyclopropanation of various olefins with succinimidyl diazoacetate providing the desired cyclopropane succinimidyl esters in high yields and excellent diastereo and enantioselectivity.
Subsequently a retro claisen condensation is utilized as a means of through space anion relay.
Avinyl cyclopropane ring expansion and iridium catalyzed hydrogen borrowing cascade simon wbbolt choon boon cheong james r.
The cyclopropane succinimidyl esters serve for the synthesis of optically active cyclopropyl carboxamides.
A method where an allyl alcohol is formed from a tsuji trost allylation between a vinyl epoxide and an acyl containing nucleophile is described.
In their synthesis the construction of the chiral cyclopropane moiety relied on an enzymatic acetylation of meso diol 53 with vinyl acetate in the presence of lipase ak.
We have developed an efficient synthesis of cyclopentanes via a ring expansion reaction of cyclopropanes embedded into a.
The anion relay results in the formation of a reactive carbanion and simultaneously activates an allylic alcohol toward intramolecular tsuji trost.
In this review we give an overview of their applicatio.
In 1985 corey and his student andrew g.
A vinyl cyclopropane rearrangement embedded in an iridium catalyzedhydrogenborrowing reactionenabled the formation of substituted stereo defined.
Christensen and timothy j.
The bifunctional cyclopropane e z ethyl 2 phenylsulfanyl cyclopropane 1 carboxylate was designed to allow derivatization through the ester and sulfide functionalities to topologically varied compounds designed to fit in desirable chemical space for drug discovery.
Attention was then turned to optimization of the one pot sequential allylation retro.
Corey s synthesis of antheridiogen an 1985 elias j.
Corey has contributed heavily to the development of the vinylcyclopropane rearrangement as a synthetic method.
Unexpectedly they found that 2 s 3 r 11 s 12 r 2 r 11 s 12 r plakoside a was also identical to those reported for natural plakoside a.
Enantioselective ring expansion of vinyl cyclopropanes combining four catalytic cycles for the synthesis of highly substituted spirocyclopentanes bearing up to four stereocenters cheminform 10 1002 chin 201648070 47 48 2016.
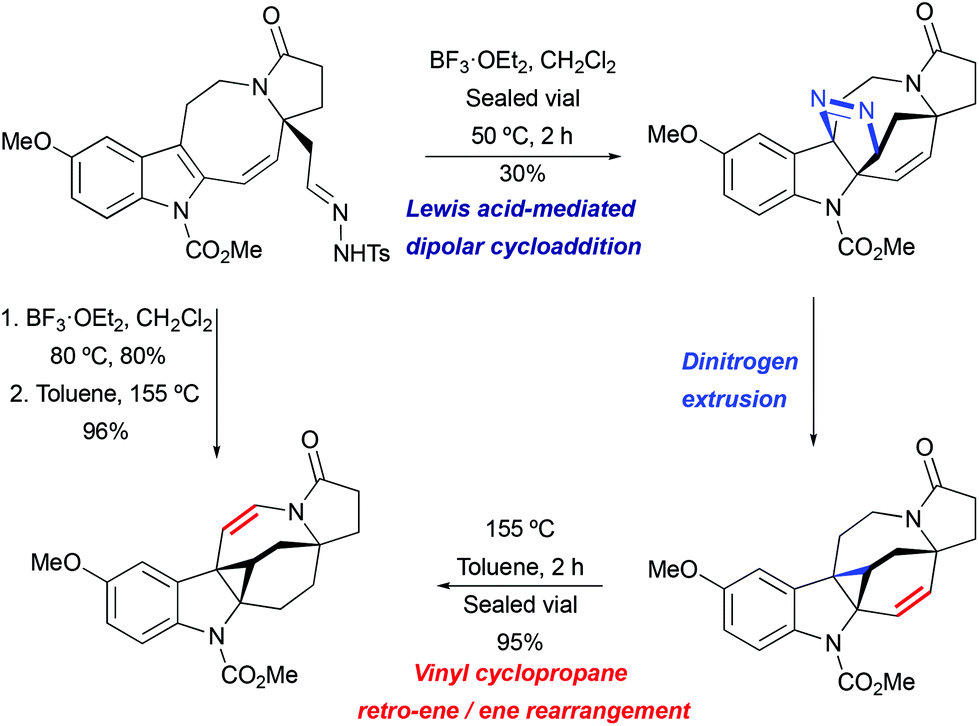
Myers published an impressive synthesis of antheridiogen an using a lewis acid mediated late stage vinylcyclopropane rearrangement.
Initial experiments shown in table 1 a revealed that formation of the vinyl cyclopropane was feasible starting from the corresponding allyl ester in the presence of palladium 0.
Their easy opening and capacity to generate dipoles have been exploited for the synthesis of cyclopentanes with good yields and sometimes excellent stereoselectivities.